Abstract
Background: Children with high-risk 1st relapse B-ALL after frontline chemotherapy have a poor prognosis and are candidates for allogeneic hematopoietic stem cell transplant (alloHSCT) after achieving complete remission (CR). We previously reported that treatment with blinatumomab, a CD3/CD19-directed BiTE ® (bispecific T-cell engager) molecule, resulted in superior efficacy in event-free survival (EFS) and minimal residual disease (MRD) remission vs intensive multidrug chemotherapy as the 3rd consolidation block before alloHSCT in children with high-risk 1st relapse B-ALL in a phase 3 RCT (JAMA 2021;325:843-54).Here we provide FU of EFS, overall survival (OS), and safety as of Sep 2020, ie, beyond the July 2019 primary analysis.
Methods: In this phase 3 RCT (Amgen NCT02393859), children >28 days and <18 years old with high-risk 1st relapse B-ALL were randomized 1:1 after induction and 2 cycles of high-risk consolidation to receive either a 3rd consolidation course with blinatumomab (15 μg/m 2/day, 4 weeks, continuous IV infusion) or intensive multidrug chemotherapy. Study enrollment required M1 (<5% blasts) or M2 (≥5% and <25% blasts) bone marrow (BM) at randomization. Stratification variables included age, BM status after the 2nd cycle of consolidation, and MRD status after induction. Patients with CR (M1 BM) after treatment could undergo alloHSCT. Endpoints included EFS (primary), OS, MRD remission, and adverse events (AEs).
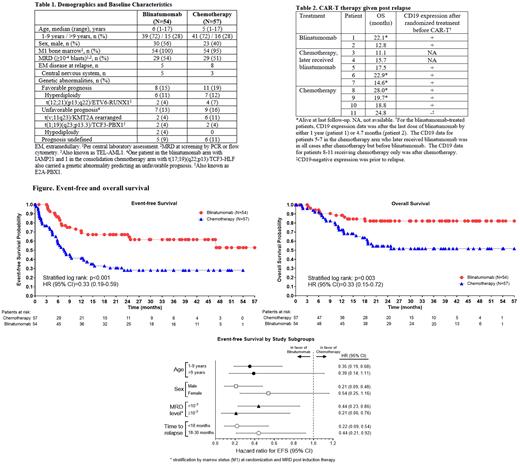
Results: Enrollment was terminated based on the interim analysis for benefit of blinatumomab (7/17/19 primary analysis). The following results reflect an updated analysis as of 9/14/20. Between Nov 2015-Aug 2019, 111 patients were randomized; 54 (49%) received blinatumomab and 57 (51%) received chemotherapy at 47 centers in 13 countries. Baseline characteristics were comparable in both arms (Table 1). After a median FU of 31 months, EFS was significantly superior with blinatumomab vs chemotherapy [63% vs 37%, stratified log-rank p<0.001, hazard ratio (HR): 0.33, 95% CI: 0.19-0.59; Figure]. The HR of EFS favored blinatumomab in all specified subgroups, including those relapsing <18 months from diagnosis (HR: 0.22, 95% CI: 0.09-0.54); OS shows a strong benefit with blinatumomab vs chemotherapy (83% vs 60%, stratified log-rank p=0.003, HR: 0.33, 95% CI: 0.15-0.72). MRD remission (<10 -4 blasts) rate was higher with blinatumomab than chemotherapy (91% [49/54] vs 48% [27/56]). AlloHSCT was achieved prior to relapse by 51/54 patients in the blinatumomab arm and 39/57 patients in the chemotherapy arm. No fatal AEs were reported. Grade ≥3 AEs were reported by 61% of patients in the blinatumomab arm [0 cases of cytokine release syndrome (CRS), 11 infections (7 occurring post alloHSCT/chemotherapy), 3 neurologic events], and 83% of patients in the chemotherapy arm [0 cases of CRS, 6 infections, 1 neurologic event]. Second relapses occurred in 16/54 (30%) and 34/57 (60%) patients and were extramedullary in 5/16 (CNS=5) and 8/34 (CNS=3) in the blinatumomab and chemotherapy arms, respectively. CD19-negative relapse was seen in 1 patient in the chemotherapy arm (1/57) and 2 patients in the blinatumomab arm (2/54) prior to any subsequent CD19-directed therapy.
CAR-T cell treatment after investigational therapy was reported for some patients; blinatumomab arm: 2/54 and chemotherapy arm: 9/57 patients (Table 2). Both blinatumomab arm patients had CD19+ disease after blinatumomab treatment, with OS of 22.1 and 12.8 months, and 1/2 alive at last FU. Of the 5 patients in the chemotherapy arm who later received blinatumomab, 3 had CD19+ disease after chemotherapy and 2 did not have CD19 expression data available; OS for these 5 patients ranged from 11.1-22.9 months with 2/5 alive at last FU. There were also 3 patients in the chemotherapy arm (but no exposure to blinatumomab) with CD19+ relapse who received CAR-T at relapse; OS for these patients ranged from 18.8-28.0 months and 2/3 were alive at last FU; a fourth patient with CD19-negative relapse who received chemotherapy only at relapse had OS of 24.8 months.
Summary/Conclusion: In children with high-risk 1st relapse B-ALL, treatment with 1 cycle of blinatumomab vs chemotherapy before alloHSCT resulted in significantly superior EFS and improved OS. The incidence of CD19-negative relapse after blinatumomab was low. No evidence of a negative impact of blinatumomab followed by CAR-T cell therapy was noted in this small subset of patients.
Locatelli: Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Miltenyi: Speakers Bureau; Medac: Speakers Bureau; Jazz Pharamceutical: Speakers Bureau; Takeda: Speakers Bureau. Zugmaier: Amgen: Current Employment; Micromet/Amgen: Patents & Royalties: Patents 20190300609 and 20130323247 licensed; receives royalties of family members of international applications published as WO2010/052014; WO2010/052013; WO2011/051307; WO2012/055961; WO 2012/062596; WO2014/122251; and WO2015/181683; Amgen: Current equity holder in publicly-traded company. Rizzari: Sobi: Other: personal fees; Shire: Other: Grants and personal fees; Medac: Other: Grants and personal fees; Jazz Pharmaceuticals: Other: Personal fees; Amgen: Other: Personal fees. Morris: Amgen: Current Employment. Gruhn: AmgenGmbh: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel costs; Bellicum Pharma Gmbh: Membership on an entity's Board of Directors or advisory committees, Other: travel costs; EUSA Pharma Gmbh: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Honoraria, Other: travel costs; Novartis Pharma Inc.: Honoraria, Other: travel costs; pfizer: Honoraria; servier: Honoraria, Other: travel costs; Neovii Biotech GmbH: Other: travel costs; medac GmbH: Other: travel costs. Zeng: Amgen: Current Employment. Pilankar: Iqvia: Current Employment, Other: Iqvia provides support for Amgen. Kormany: Amgen: Current Employment. Moericke: Amgen: Other: Grants; Shire: Other: Personal fees. Peters: Amgen: Membership on an entity's Board of Directors or advisory committees, Other: Travel grants. Saha: Amgen: Other: Personal fees. von Stackelberg: Amgen: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Jazz: Consultancy, Honoraria; Miltenyi: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal